-scaled.jpg)


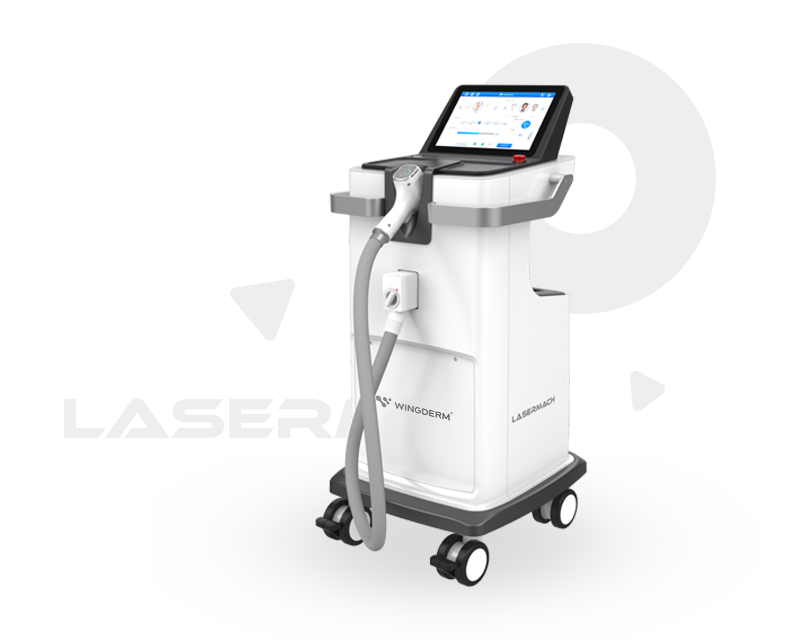
Lasermach is suitable for all skin types, non-invnsive, no down time, economical and reliable, and laser hair removal is considered as a gold standard for hair removal.

-scaled.jpg)

Wingderm® announces that its Renuva 1550nm non-ablative fractional laser system has obtained MDR certification in the European Union, marking a significant regulatory milestone for the device within the EU market.
Renuva is certified under MDR for treating acne scars and striae across Fitzpatrick skin types I to VI. Before this approval, Renuva already held MDD certification in the EU. Moving from MDD to MDR reflects compliance with the latest regulatory standards for medical devices. Renuva’s MDR certification provides partners with a clear compliance basis.
Renuva utilizes fractional photothermolysis technology, delivering controlled energy into micro-areas of the skin while preserving surrounding tissue. This approach aims to enhance the skin’s appearance, supporting a comfortable and minimally interruptive beauty experience. Renuva is designed for professional settings and to allow clients to resume their daily activities with minimal interruption. Moreover, Renuva procedures can be adjusted according to professional guidance for safe operation on different skin types.

Specialists have shared many observations from Renuva procedures. “Renuva can be combined with other aesthetic procedures or used on its own within a treatment program. Individuals typically resume normal activities soon after each session, with the experience being described as well-tolerated.” said Dr. Martyn King, Vice-chair of the Joint Council of Cosmetic Practitioners and member of the British College of Aesthetic Medicine. “Renuva treatment is frequently chosen by male clients, as the experience is generally described as comfortable, with limited disruption to daily life.”
Renuva’s MDR certification reinforces its market position and provides clinics with a regulated, technology-based option for professional aesthetic programs, empowering beauty professionals to offer innovative, safe, and customizable skin enhancement experiences.









Mesoskin, the non-invasive virtual mesotherapy device for skin and scalp care, has been applied in more than 1.1 million procedures worldwide, marking a significant milestone in its journey of innovation and trust.
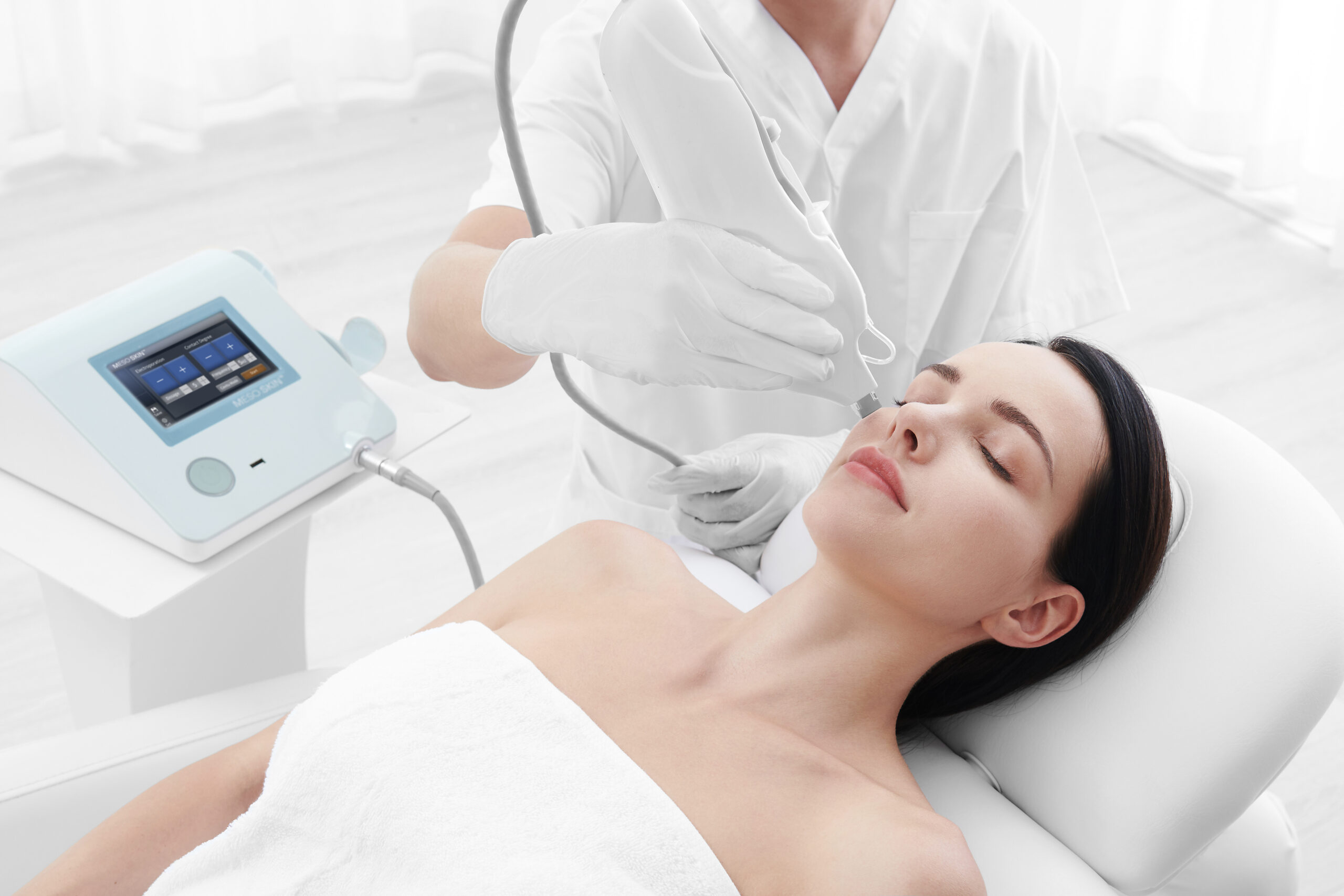
Mesoskin was developed by Wingderm® and launched in 2017. By offering a gentle, non-invasive approach, it is designed to assist the absorption of active ingredients in skincare and support skin care and scalp care routines. Since its launch, Mesoskin has won widespread praise and continues to be highly recommended by aesthetic practitioners.
Prof. Hang Wang, Executive Chairman of AMWC China, observed the use of Mesoskin in professional settings. She noted that Mesoskin assists with the application of high-molecular-weight cosmetic ingredients in a gradual and controlled manner. Unlike directly applying products or using tools such as a derma pen, Mesoskin’s cartridge tip is designed to cover a larger area, helping beauticians apply cosmetic products efficiently while maintaining a comfortable experience. With its non-invasive design and versatility, Mesoskin is now regularly used in beauty routines and salon care.

Mesoskin pairs easily with various cosmetic products, allowing for customized skincare. “I have been using Mesoskin for many years, my clients often comment that their skin feels well hydrated and looks more radiant after sessions” said Dr Gabriela Mercik, one of London’s top aesthetic practitioners.
“Mesoskin’s global application reflects its strong presence in the aesthetic industry and the continued demand for safe and comfortable aesthetic experiences. Wingderm continues to drive its growth through innovation, expanding and refining its product lineup. By introducing forward-looking solutions, Wingderm® will bring value to global partners and contributes to the progress of the aesthetic industry. ” said Will Wang, CEO of Wingderm®.

Wingderm®, a leading innovator in energy-base medical aesthetic technologies, officially made its debut at The Aesthetic Show (TAS) 2025, held from June 27 to 29 in Las Vegas. This marks a key step as the brand grows in the U.S. medical aesthetic market.

At TAS, the Wingderm USA team led all product presentations and customer engagement activities, providing on-site, real-time technical consultation. This local setup eliminated the typical barriers of time zones and overseas support, offering a seamless experience for attendees. More than being present, the U.S. team brought cultural fluency and operational agility, building closer and more effective connections with practitioners.


The two featured energy-based devices meet the needs of North American practitioners for non-invasive treatments and suitability across diverse skin tones. Both have received FDA clearance, which helps build trust and supports their acceptance in the U.S. market. The FDA clearance not only affirms Wingderm®’s technology, but also lays the foundation for serving clients and building long-term operations in the U.S. market.

Wingderm USA’s participation in the show was led by CEO Carol Ren, whose deep understanding of industry trends and Wingderm®’s technologies earned widespread trust. At the booth, she shared in-depth knowledge of the products’ features and their clinical applications. Carol said, “The show is just the start. The real value comes from ongoing service. Wingderm USA will act as a strategic hub, linking our headquarters to the U.S. market and providing steady, local support to our clients.”


From expanding global distributor network to having its U.S. operations become fully operational, Wingderm® is transforming from brand expansion to building a lasting presence in key markets like North America. This time, we’re not just here—we’re here to stay, serve, and support our partners’ success.

On May 22, 2025, the “Sino-EU Exchange Forum on New Quality Productive Forces” was held in Zurich, Switzerland. The event was hosted by the Haidian District People’s Government of Beijing Municipality, and co-organized by Beijing Zhongguancun Science City Innovation Development Co., Ltd., Beijing Haikai Holdings (Group) Co., Ltd., Legend Holdings, and China Europe International Business School (CEIBS) . The forum was attended by many delegates from the political, business, and academic sectors across China and Europe, including Chen Yun, Consul General of China in Zurich and Liechtenstein; Zhang Ge, Party Secretary of Haidian District; and Lukas Huber, Managing Director of The Greater Zurich Area Ltd. (GZA).

(From left to right: Chen Yun, Consul General of China in Zurich and Liechtenstein; Zhang Ge, Party Secretary of Haidian District; Lukas Huber, Managing Director of The Greater Zurich Area Ltd. (GZA))
Will Wong, Founder and CEO of Wingderm®, joined the event as a representative of Haidian-based tech companies. During the forum, he signed a long-term strategic cooperation agreement with SWISS clinitech sàrl. They will work closely together to drive the expansion of innovative aesthetic medical devices in the European market. Zhang Ge, Party Secretary of Haidian District; Cheng Wanhui, Director of Industry Division III at the Zhongguancun Science City Administrative Committee; and Xin Guo, Mayor of Dongsheng Town in Haidian District, were present at the signing ceremony.


(From left to right: Dai Lian, Deputy General Manager of Beijing Zhongguancun Science City Innovation Development Co., Ltd.; Xin Guo; Zhang Ge; Will Wong; Ivan Adam, General Manager at Swiss Clinitech Sàrl; and Cheng Wanhui)


Will Wong said, “Wingderm® is a high-tech company based in Haidian. We’re grateful for the strong support from the Haidian government, which has enabled us to bring our innovations to the global stage. We’ll continue to build on our R&D strengths and work closely with our Swiss partner to promote high-quality medical aesthetic devices worldwide.”

From June 6 to 8, 2025, IMCAS Asia was successfully held in Bangkok, Thailand. As one of the world’s leading medical aesthetic events, IMCAS Asia brought together the latest developments and trends in the Asian medical aesthetic market. Wingderm® once again showcased its innovative medical aesthetic solutions.

Focusing on Asia’s Growing Medical Aesthetic Market
The Asian market includes both mature economies, such as China, Japan, and South Korea, as well as rapidly growing Southeast Asian countries. This makes the region a crucial player in the global medical aesthetic industry. IMCAS Asia is not only a platform for sharing knowledge, but also offers Wingderm® a valuable opportunity to better understand practitioner perspectives and stay aligned with market trends, supporting product upgrades and strategic expansion.


Tech Meets Trust: Driving Mutual Growth through Innovation
At IMCAS Asia, Wingderm® presented a series of advanced medical aesthetic devices, including Mesoskin for non-invasive delivery, Lasermach for fast and comfortable laser hair removal, and Renuva, a device powered by 1550nm non-ablative fractional laser for skin resurfacing. Our success at the event was thanks to the support of our local distributor in Thailand. We will continue this close partnership for mutual benefit.


Next Stop: TAS 2025 in Las Vegas
Driven by technology and innovation, Wingderm® is always looking for new ways to apply our technologies and enhance user experiences. Through industry events, we share our latest advancements and strengthen connections with industry professionals around the world. Wingderm® will be at booth 712 during TAS 2025, held from June 27 to 29 in Las Vegas. Please visit us there.

Wingderm® once again participated in the 2025 AAD Annual Meeting, which was held in Orlando, Florida, from March 7 to 9. The American Academy of Dermatology is the largest, most influential, and most representative dermatologic association. During the event, attendees had the opportunity to discover the latest technological innovations from Wingderm® and their applications.


Innovative Laser Technologies for Superior Results
Wingderm® showcased its advanced laser devices. Lasermach features 755nm, 808nm, and 1064nm wavelengths, effectively targeting melanin at different follicle depths for optimal and long-lasting results. The 1550nm non-ablative fractional laser, equipped with advanced optical scanning tracking technology, penetrates deep into the skin without damaging the epidermis, stimulating collagen regeneration for skin resurfacing. These proven technologies have gained widespread recognition.
Wingderm® offers one-on-one consultations, addressing key questions about the devices, such as performance and maintenance. In addition, by simulating the treatment process, visitors gain a more intuitive understanding of the device’s value and technological advantages, further strengthening their confidence in decision-making.




To strengthen the presence in North American market, Wingderm USA Inc. was established in California, ensuring faster response times and seamless customer support.
“As demand for our products in the U.S. market continues to grow, we plan to showcase our products at more exhibitions in the U.S. We believe that through ongoing innovation and brand promotion, Wingderm® will strengthen its position in the U.S. and global markets.” said Carol Ren, CEO of Wingderm USA Inc.

Wingderm® once again participated in IMCAS World Congress from 30th January to 1st February 2025, presenting its core devices and newest platforms. The showcased devices included Lasermach Duo, Lasermach, Renuva, Mesoskin, and and other advanced solutions.

Wingderm®’s Booth Bustling with Visitors
Wingderm®’s booth was constantly bustling with visitors, drawn by professional device presentations and in-depth technical discussions. Industry experts and quality-focused practitioners were eager to engage and exchange ideas. The interaction helped them gain a deeper understanding of Wingderm®’s brand concept and device advantages.





Lasermach Duo: A Milestone in Laser Hair Removal Innovation
Lasermach Duo, the newest generation of laser hair removal solution, offers effective treatments for all hair types and skin tones with three wavelengths. Dual handpieces with an innovative connection structure for easy installation, combined with an enhanced cooling system, enable practitioners to achieve excellent clinical results while providing beauty seekers with efficient and comfortable treatments. Lasermach Duo is equipped with WiCAN (Wingderm® Intelligent Connection CAN Bus), an embedded underlying interconnection platform, which enables seamless data interaction and remote control. Lasermach Duo is a great addition to medical spas and clinics, whether they have been operating for years or are just starting out.


New Radiofrequency(RF) Device Preview
At the “Tech-Driven Beauty” conference, Wingderm® unveiled its latest breakthrough in RF technology, marking the company’s official entry into the RF field. The device, currently in development, integrates cutting-edge RF technology to deeply stimulate collagen regeneration, providing firming and anti-aging effects. During the conference, attendees had the opportunity to learn more about the RF device’s design and technology, and they expressed great anticipation for its market value.


About Wingderm®
Wingderm® since its establishment in 2016, with the aim of “Aesthetics & Technology, Easy to Achieve”, provides leading and reliable intelligent photoelectric medical aesthetic devices, which have been exported to more than 80 countries, with over 15,000 units installed, recognized for safety and effectiveness by experts and beauty seekers.


















 Many visitors stopped by Wingderm®’s booth and stayed for valuable insights. Wingderm® members communicated and discussed with peers, industry experts and business representatives from all over the world, introducing the design concepts and positionings of the products, staying current with the latest industry and technology trends, learning the successful experience of many outstanding companies. Through communication with all of them, Wingderm® can design the strategies of R&D and market positioning more accurately.
Many visitors stopped by Wingderm®’s booth and stayed for valuable insights. Wingderm® members communicated and discussed with peers, industry experts and business representatives from all over the world, introducing the design concepts and positionings of the products, staying current with the latest industry and technology trends, learning the successful experience of many outstanding companies. Through communication with all of them, Wingderm® can design the strategies of R&D and market positioning more accurately.


 Throughout the exhibition, Derma Innovation, Wingderm®’s distributor in Thailand, has provided substantial support and assistance. With the local advantages of Derma Innovation, Wingderm® can communicate with visitors more effectively, enhancing their trust and recognition of Wingderm®’s products and services.
Throughout the exhibition, Derma Innovation, Wingderm®’s distributor in Thailand, has provided substantial support and assistance. With the local advantages of Derma Innovation, Wingderm® can communicate with visitors more effectively, enhancing their trust and recognition of Wingderm®’s products and services.
 At every international exhibition, Wingderm® presents professional products and advanced aesthetic concepts, while also improving the reputation. In the future, Wingderm® will continue to participate in international exhibitions and industry events, and look forward to working with excellent partners to provide high-quality products and services for global beauty seekers.
At every international exhibition, Wingderm® presents professional products and advanced aesthetic concepts, while also improving the reputation. In the future, Wingderm® will continue to participate in international exhibitions and industry events, and look forward to working with excellent partners to provide high-quality products and services for global beauty seekers.
