Wingderm®, a leading innovator in energy-base medical aesthetic technologies, officially made its debut at The Aesthetic Show (TAS) 2025, held from June 27 to 29 in Las Vegas. This marks a key step as the brand grows in the U.S. medical aesthetic market.

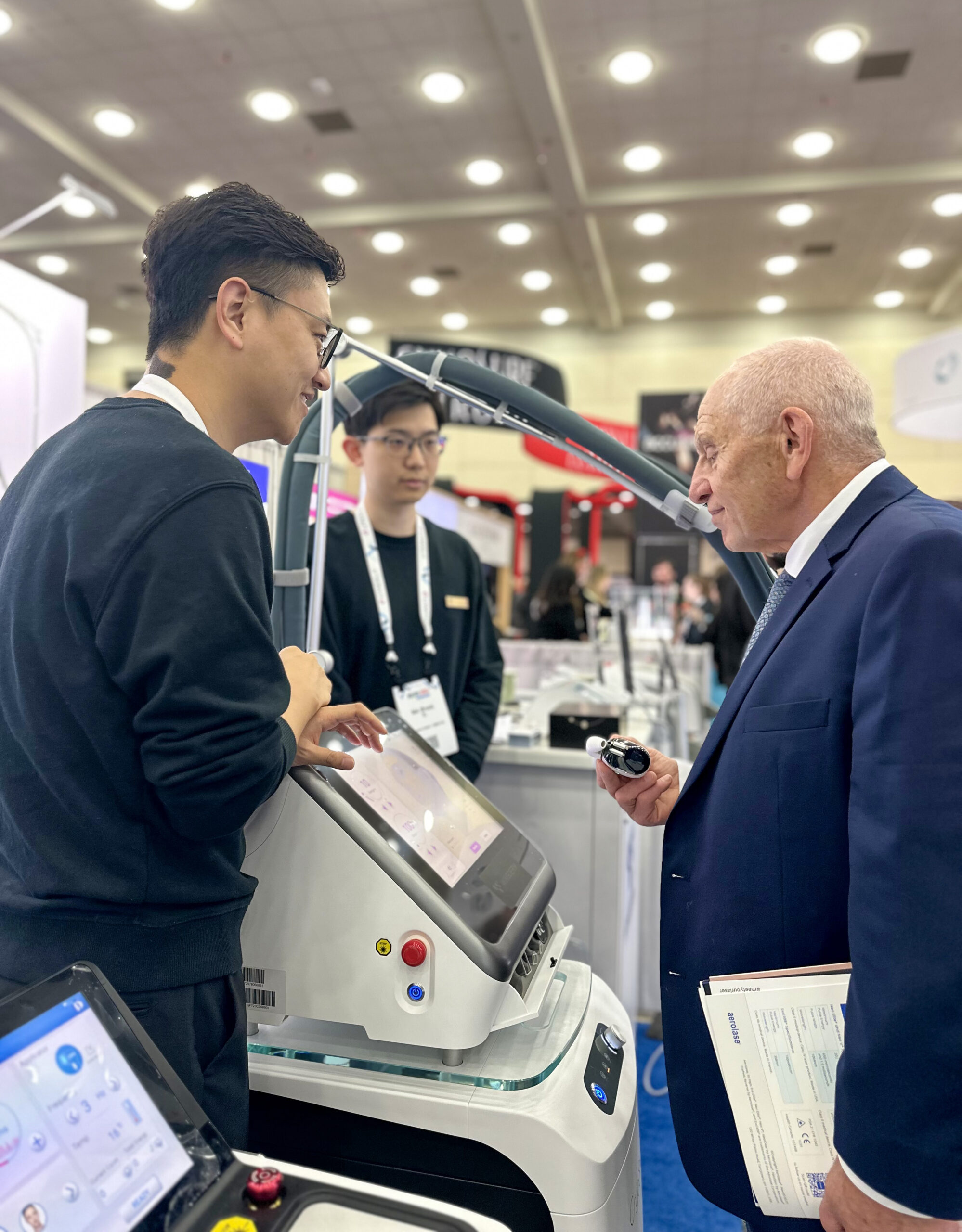
At TAS, the Wingderm USA team led all product presentations and customer engagement activities, providing on-site, real-time technical consultation. This local setup eliminated the typical barriers of time zones and overseas support, offering a seamless experience for attendees. More than being present, the U.S. team brought cultural fluency and operational agility, building closer and more effective connections with practitioners.


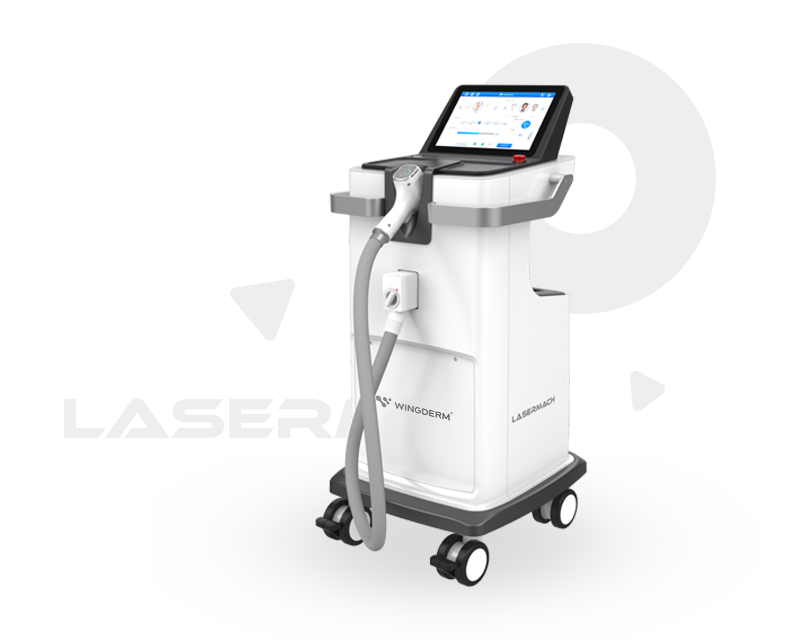
The two featured energy-based devices meet the needs of North American practitioners for non-invasive treatments and suitability across diverse skin tones. Both have received FDA clearance, which helps build trust and supports their acceptance in the U.S. market. The FDA clearance not only affirms Wingderm®’s technology, but also lays the foundation for serving clients and building long-term operations in the U.S. market.

Wingderm USA’s participation in the show was led by CEO Carol Ren, whose deep understanding of industry trends and Wingderm®’s technologies earned widespread trust. At the booth, she shared in-depth knowledge of the products’ features and their clinical applications. Carol said, “The show is just the start. The real value comes from ongoing service. Wingderm USA will act as a strategic hub, linking our headquarters to the U.S. market and providing steady, local support to our clients.”


From expanding global distributor network to having its U.S. operations become fully operational, Wingderm® is transforming from brand expansion to building a lasting presence in key markets like North America. This time, we’re not just here—we’re here to stay, serve, and support our partners’ success.